Revolutionising Vaccine Manufacturing for Developing Countries
Future Vaccine Manufacturing Research Hub
Collaborative and international research is the key to realising the next generation of vaccines and enabling global equitable access to them.
Professor Robin Shattock, Head of Mucosal Infection and Immunity within the Department of Infectious Disease at Imperial College London
Years of basic and applied research on vaccine manufacturing have supported the scientific community’s robust and rapid response during the Covid pandemic. Within months of the pandemic’s beginnings, a number of safe and effective vaccines were designed and trialled. However, more equitable access to vaccines for a broad range of existing pathogens are needed.
Collaborative and international research is helping to realise the next generation of vaccines, and innovative vaccine platform technologies will herald more flexible manufacturing against various existing and emerging pathogens in the most in-need demographics around the world.
Since 2018, led by Imperial College London, the Future Vaccine Manufacturing Research (FVMR) Hub has researched innovative vaccine manufacturing approaches for pathogens that impact developing countries, such as rabies, influenza, chikungunya virus, hepatitis E and SARS-CoV-2.
The FVMR Hub supports international partnerships focusing on vaccine manufacturing intensification and has completed training events for vaccine manufacturing staff from developing countries on a number of manufacturing topics including the use of Quality by Design (QbD).
Vaccine manufacturing staff from countries such as Bangladesh, Vietnam, China and Uganda have completed training in labs within the UK, supporting tech transfer and stronger know-how to low and middle-income manufacturers. Transparent and open working teams are key and embody the ethos of the FVMR Hub.
Exemplar outputs from the FVMR Hub include increased understanding of the design and production of outer-membrane vesicle-based vaccines, enhanced manufacturing processes for RNA vaccines, improved downstream processing of innovative yeast strain platform technologies, and translation of a novel RNA-based vaccine candidate against SARS-CoV-2 through to phase I clinical assessment in Uganda.
The FVMR Hub members have published in open access outlets, including journals, news/press releases, and volunteered in educational workshops to engage with the general public. In addition to applying innovative approaches to vaccine manufacturing processes, improving the general public’s understanding and appreciation of vaccine manufacturing is a main target for the FVMR Hub.
Learn more at www.imperial.ac.uk/future-vaccine-hub
Success Stories
1. Hub members have modelled vaccine manufacturing based on RNA as well as adenoviral technologies. The Hub has shown how bioreactor production rates, lead times and scale, and their impact on cost, influence the manufacturing scales and outputs capable for these technologies. Policymakers and vaccine manufacturers may use this blueprint to inform strategies for future epidemics or pandemics.
2. Hub members have visualised SARS-CoV-2, the causative agent of Covid, to determine that linoleic acid binds efficiently to the closed form of the virus spike glycoprotein trimer. This work elucidated the virus interactions with natural fatty acids, offering insights into its interactions during infection and pathology and novel vaccine targets.
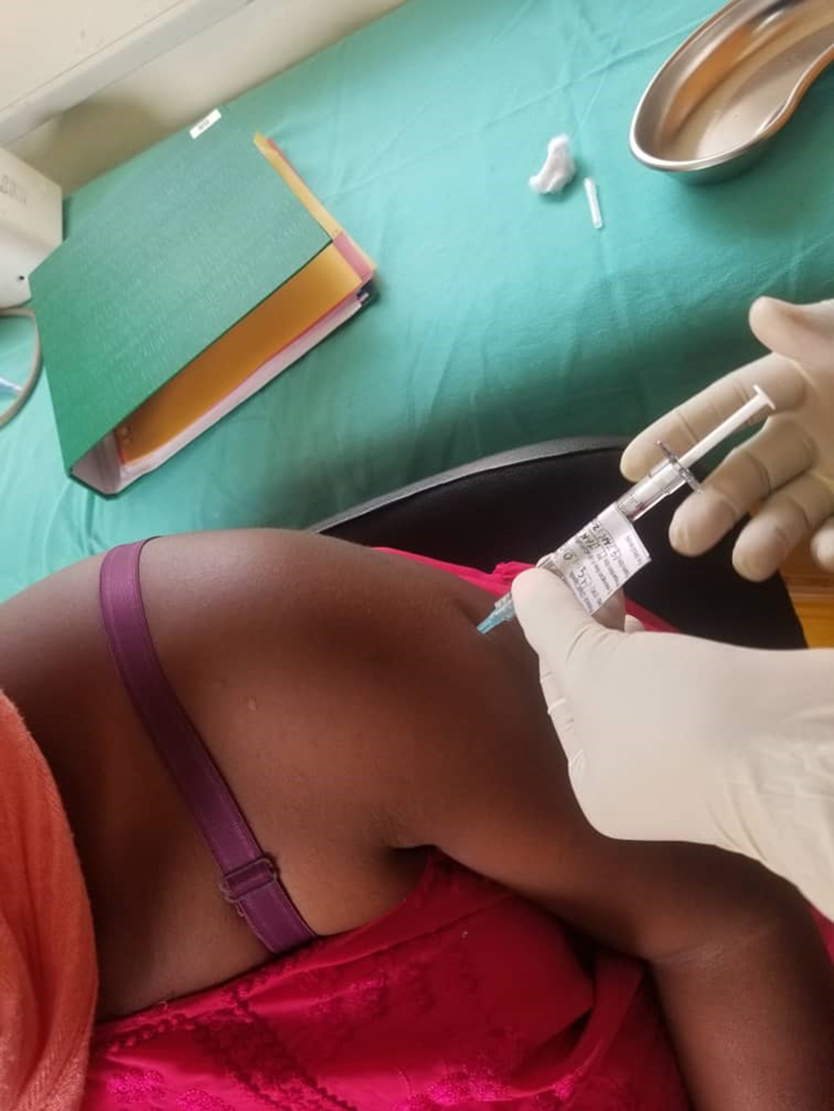
3. Fully funded by the Hub, Imperial College London and MRC/Uganda Viral Research Institute are conducting a Phase I trial of an innovative RNA-based vaccine candidate against SARS-CoV-2. Informed by a trial of a first-generation vaccine candidate, COVAC Uganda is investigating a second-generation design hypothesised to dampen inflammation following vaccination. The trial will quantify safety and immunogenicity for participants that are seronegative and seropositive for antibodies against the virus.
“Vaccine manufacturing staff from countries such as Bangladesh, Vietnam, China and Uganda have completed training in labs within the UK”
Above: The first vaccination within COVAC Uganda: the first ever clinical trial of a RNA vaccine technology in Uganda and the first trial of an saRNA vaccine technology in all of Africa
Below: The self-amplifying RNA Covid vaccine investigated in the clinical trial COVAC Uganda arrives on sit