Continuous Manufacturing & Advanced Crystallisation – Transforming Medicines Manufacturing
CMAC Future Manufacturing Research Hub
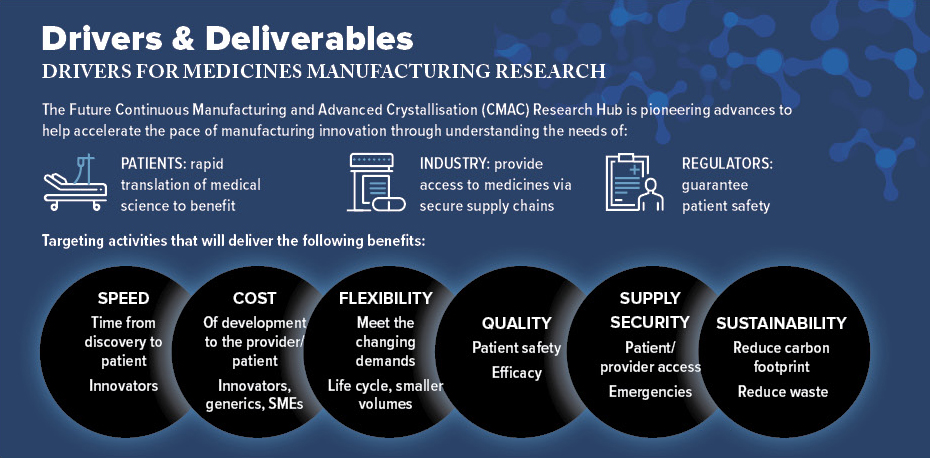
The transformation of how we develop and manufacture medicines has never been more important if we are to address pandemic preparedness, supply chain resilience, the ageing population, the urgency for net zero and realise the economic and social benefits of a robust, sustainable medicines manufacturing sector, able to rapidly translate breakthroughs in medical science to patient benefit.
By Prof. Alastair Florence, Distinguished Professor in Pharmaceutical Sciences at the University of Strathclyde
The Covid pandemic has highlighted the need to invest in resilient, productive and flexible medicines manufacturing and supply chains. The climate crisis also invites the international community to find ways to achieve net zero emissions.
Medicines manufacturing has to adapt to meet these challenges head-on. If the UK is to grow our manufacturing base and become a global leader in medicines development and manufacturing, we urgently need to make them in more efficient, responsive and resilient ways.
Medicines manufacturing is a key sector for the UK, generating exports of more than £25bn with the highest GVA of any sector (£8.5bn). The industry invests over £4bn per annum on R&D in the UK. Globally, the medicine market is projected to grow at 3% to 6% CAGR, with the total market reaching £1.2tr by 2025.
Process development and manufacture to supply materials for clinical testing is costly, being nearly 20% of the total R&D cost per successful drug, estimated at up to US$3bn. Medicines costs are an increasing burden to the NHS budget (£16bn increasing at ~7% per annum). Our research, in partnership with industry, seeks to deliver the underpinning know-how, technology and talent pipeline to transform the way we develop and make medicines.
The Medicines Manufacturing Industry Partnership (MMIP) in the UK along with the US FDA have identified advanced manufacturing technologies including continuous manufacturing and industrial digital technologies (IDTs) as important solutions that can lead to cost-effective, sustainable and secure access to quality medicines.
IDTs include technologies such as robotics; automation; artificial intelligence and analytics; simulation; augmented and virtual reality; cloud-based platforms, and additive layer manufacturing. They all rely on access to clean, accessible, quality data and we are working collaboratively to enable data-driven research and innovation.
The lack of appropriate skills in the workplace is identified as the main barrier for translation and adoption of disruptive, innovative advanced and digital medicines manufacturing technologies.
In addition to our Hub focus on Quality by Digital Design, innovative development DataFactories, Digital Twins and modular continuous MicroFactories, CMAC has a leading training programme serving the medicines manufacturing talent pipeline. This integrated approach to research and training will deliver the long-term skills needed by industry to enable the workforce of tomorrow for sustainable, cost-effective and flexible supply of medicines to patients.
Learn more at www.cmac.ac.uk
Our Hub portfolio includes:
1. Digitalisation of Chemistry, Manufacturing and Control via IDTs.
2. Quality by Digital Design framework that digitalises Quality by Design exploiting modelling and data-driven decision support tools.
3. The Autonomous Crystallisation Classification DataFactory will deliver large structured data sets for interrogation by image.
4. Analysis and machine learning.
5. Digital Twins of key processes are being developed alongside MicroFactory platforms.
6. Future Workforce will be highly skilled, augmented and ‘industry ready’ to use cutting-edge IDTs and will include future research leaders for industry and academia.
7. Translation of research assets to higher TRL with the goal of accelerating this process to become more agile and responsive.