A Golden Age for Healthcare Innovation
Sectors: Medical Devices
Above: Genedrive is helping prevent hearing loss in premature babies, allowing for the correct antibiotic to be given within the ‘golden hour’. Credit: Genedrive
From artificial intelligence and machine learning to genetics and new materials, the investments made during the past two years are paying bumper dividends for the British medical devices sector.
Charles Orton-Jones
Above: Panakeia’s approach is significantly faster than existing tests, providing results in a few minutes rather than days or weeks. Credit: Panakeia
The confluence of so many breakthrough technologies at once is producing a rich haul of sophisticated new machines. Designers today can call on artificial intelligence, machine learning, internet connectivity, nanotechnology, new materials such as graphene, and cheap DNA decoding technology. It’s boom time.
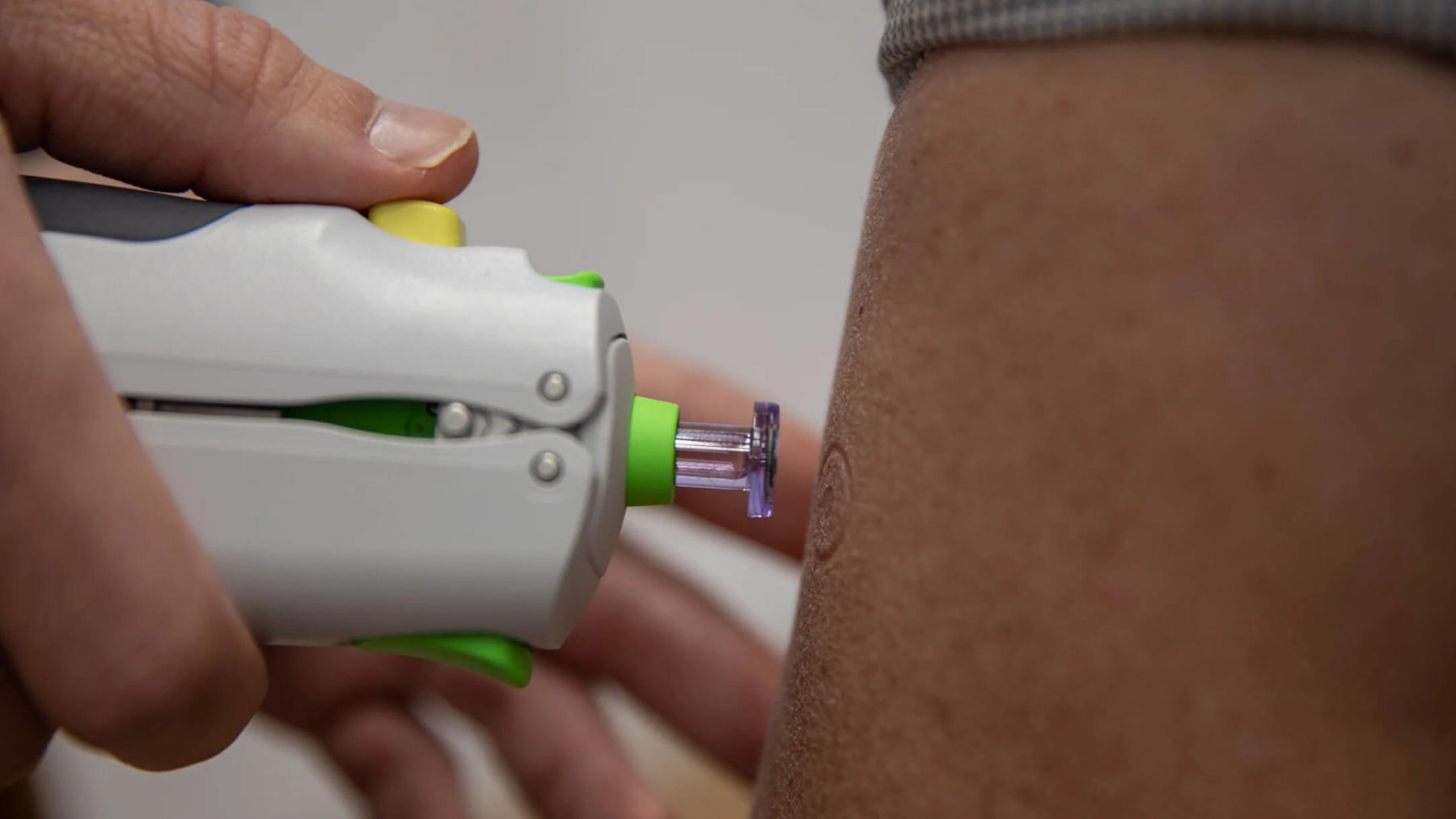
A classic example is University of Manchester start-up Genedrive. The company is on the brink of tackling deafness in premature babies. Taking just 25 minutes, Genedrive’s bedside machine identifies whether a critically ill baby has a gene that could result in permanent hearing loss if they are treated with a common emergency antibiotic.
People admitted to intensive care are usually given an antibiotic called Gentamicin within 60 minutes. While Gentamicin is used to safely treat about 100,000 babies a year, one in 500 babies carries the gene that can make it cause permanent hearing loss. The new test means that babies found to have the genetic variant can be given an alternative antibiotic within the ‘golden hour’.
The new swab test technique would replace a test that traditionally took several days and could save the hearing of 180 babies in England alone each year. Furthermore, the test could save the NHS £5m annually by reducing the need for other interventions, such as cochlear implants. Trials are underway at two Manchester hospitals.
AI-powered breakthroughs
AI diagnostics is huge right now. Cambridge-based Panakeia analyses digital images of tumour samples. An artificial intelligence engine examines the digital file to identify any anomalies and recommends the best treatment in just 15 minutes.
Founder Pahini Pandya says her approach is “immensely faster than existing tests, providing a diagnostic readout from the original H&E [haematoxylin and eosin] image in a few minutes, as opposed to a few days or even weeks”. The low cost means accurate cancer diagnoses will be available in poorer global regions.
Another AI pioneer in Cambridge is focusing on the 83 million people with cardiovascular disease. ElectronRx harnesses smartphone sensors to gather data which algorithms then examine to identify health issues that require medical interventions. The company employs 22 staff and aims to turn smartphones into frontline medical devices.
In Oxford, a leader in AI is Optellum. Its product is the first AI Clinical Decision Support software for lung cancer management. The AI examines clinical data to offer early diagnosis and make treatment suggestions. Optellum claims to possess the world’s largest dataset of CT scans in order to educate its cancer-analytics AI engine.
The effects of Covid-era investments
are also being felt. DIOSynVax is led by Professor Jonathan Heeney, Head of the University of Cambridge’s Laboratory of Viral Zoonotics. It produces a remarkable needle-free vaccine able, in theory, to combat all variations via an air-gun device that blasts the vaccine on the skin. If successful, it could be scaled up and manufactured as
a powder to boost global vaccination efforts, particularly in low- and middle-income countries.
“As new variants emerge and immunity begins to wane, we need newer technologies. It’s vital that we continue to develop new generation vaccine candidates ready to help keep us safe from the next virus threats,” Professor Heeney explains.
“These next-generation DIOSvax vaccines should protect us against variants we’ve
seen so far – alpha, beta, delta variants, for example – and hopefully future-proof us against emerging variants and potential coronavirus pandemics.”
In March 2022, DIOSynVax secured £32m from the Coalition for Epidemic Preparedness Innovations (CEPI) to undertake proof-of-concept preclinical studies and initial clinical development.
Regulation becoming more onerous
Medical devices which work at the home are growing in popularity, post-pandemic. Dr Chris Vincent, of design and innovation agency PDD, is a keen observer of industry trends.
“While remote methods in healthcare were introduced long before the emergence of the Covid pandemic, the past two years inevitably accelerated and changed the conversation around them, as the industry has had to evaluate the safest way to test and assess medical devices and services,” he notes.
Almost every aspect of medical devices is affected. “From home test kits and home injections to dialysis machines and working with pharmacies remotely on setting dosing limits, it’s been inspiring to watch the coming together of everyone to deliver safe and effective testing and treatment while ensuring the needs of the patient and home user remain at the centre of any changes.”
There’s also the issue of regulation. Innovation may be desirable, but EU and UK legislation remain onerous. And only getting tighter. Bob Tilling, VP of global sales at compliant labelling specialist, Kallik, says companies have been adjusting to the 2021 EU Medical Devices Regulation, and now have the IVDR (vitro diagnostic medical devices) to contend with.
He says there are new requirements ahead; “Looking at regulation at the national level, we can also expect compliance efforts to be duplicated due to the introduction of the UKCA product marking for the UK market, scheduled to replace existing CE markings from July 2023.
“If firms already have every device marked differently for sale in the US and EU markets, this will have to be duplicated again if they wish to continue selling their products in the UK.” Regulation will dominate conversations in the industry for the foreseeable future.
Frontline innovation
Naturally, none of these inventions would be possible without funding. A new £260m state fund for medical devices is already issuing cash. Randox Laboratories, made famous by its Covid testing kits, is building a new large-scale manufacturing facility in Northern Ireland with state backing.
Ortho Clinicals Diagnostics UK will expand its biological diagnostic product lines at its Pencoed site in Wales. Piramal Healthcare is undertaking facilities upgrade at its Morpeth site in Northumberland, where it develops, manufactures and packages a broad range of medical products.
Of course, not every breakthrough is high-tech. Some of the best new medical products are the result of clever thinking.
Despairing at the inefficiency of ad-hoc kits given to the 86,000 healthcare workers who visit patients at home across the UK, two community nurses have devised a better solution. Their Community Kit Bag – nicknamed a ‘mobile equipment cupboard’ – packs all required medicines and kit in colour-coded compartments that make them easy to sort and store.
A pilot study found the bag allowed nurses to see 30% more patients, due to needing to make fewer resupply trips, on top of substantial annual fuel savings. From the initial trial, there are now more than 1,200 Kit Bags in use in NHS Trusts around the UK, from Tyneside to the Isle of Wight, with thousands more
in production. l
How Far Are We From Human Digital Twins?
“Digital twins are already being used by the healthcare industry. These digital representations of human physiology can allow researchers to study the impact of diseases, drugs and medical devices and effectively bring life-saving innovations to the market in a more cost-effective, safer and quicker way.
“Arguably, if you can twin a machine, you can twin some parts of the human body and there is a huge amount of research being carried out to test the possibilities, including creating patient-specific treatment plans by determining the physiological characteristics of an individual. However, the main barrier to progress is the immense amount of computation needed and the trust required from
end-users.
“Thinking about the computing power that running such predictive models will take, we’re not yet at a stage where we can roll anything out at scale. However, it’s not incredible to believe that this is on the horizon. We should have a solution to this issue that goes beyond quantum computing in the next two to three years that will make digital twins in healthcare not only a reality but an everyday data-driven model.”
Bipin Bhaskar, chief strategy officer at technology services company Persistent Systems
News in Brief
- University of Cambridge spin-out HexagonFab creates tools to unlock the secrets of proteins and their interactions. The company recently raised £1.9m in seed funding to launch Bolt, an analytical lab instrument to speed discovery and production of next-generation biopharmaceuticals.
- Drive DeVilbiss Healthcare, which has a production facility and head office in Halifax, combined forces with GNG, a Wakefield supplier of foam mattresses, to provide ongoing philanthropic support for hospitals in Ukraine. The first consignment of 400 beds and mattresses was coordinated by charity MEDAID-4KIDS.
- The National Robotarium will open at Heriot-Watt University, in partnership with the University of Edinburgh. Home to world-leading experts in data analytics, the National Robotarium is exploring collaborative interaction between humans, robots and their environments, translating cutting-edge research into new technologies, including medical devices.
“Cambridge-based Panakeia analyses digital images of tumour samples. An artificial intelligence engine examines the digital file to identify any anomalies and recommends the best treatment in just 15 minutes”
“Medical devices which work at the home are growing in popularity”
Above: Credit HexagonFab
Above: The Community Kit Bags allow nurses to see 30% more patients. Credit: NTH Solutions

![Genedrive - RNR1 - Static Images_08[71] cartridge and system](/wp-content/uploads/2022/05/Genedrive-RNR1-Static-Images_0871-cartridge-and-system-800x482.jpg)